Difference between revisions of "Sulfuric acid"
(→External links) |
|||
| (43 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Chembox |
| − | + | | Name = Sulfuric acid | |
| − | | | + | | Reference = |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| IUPACName = Sulfuric acid | | IUPACName = Sulfuric acid | ||
| − | | OtherNames = Oil of vitriol | + | | PIN = Sulfuric acid |
| + | | SystematicName = Sulfuric acid | ||
| + | | OtherNames = Battery acid<br>Dihydrogen sulfate<br>Oil of vitriol<br>Spirit of vitriol<br>Sulphuric acid | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Smw1.png | ||
| + | | ImageSize = 250 | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageCaption = Structure of sulfuric acid | ||
| + | | ImageFile1 = | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| − | | | + | | 3DMet = |
| − | + | | Abbreviations = | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | | + | |
| SMILES = OS(=O)(=O)O | | SMILES = OS(=O)(=O)O | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
}} | }} | ||
| Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| − | | | + | | AtmosphericOHRateConstant = |
| + | | Appearance = Colorless oily liquid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = 337 | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = (above 300 °C slowly decomposes) | ||
| + | | Density = 1.84 g/cm<sup>3</sup> | ||
| + | | Formula = H<sub>2</sub>SO<sub>4</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| MolarMass = 98.079 g/mol | | MolarMass = 98.079 g/mol | ||
| − | | | + | | MeltingPt = |
| − | + | ||
| − | + | ||
| MeltingPtC = 10 | | MeltingPtC = 10 | ||
| − | | | + | | MeltingPt_ref = |
| − | | | + | | MeltingPt_notes = |
| − | | | + | | Odor = Odorless (air above it may feel dry due to its strong hygroscopicity) |
| − | | | + | | pKa = −3;1.99 |
| − | | VaporPressure = 0.001 mmHg ( | + | | pKb = |
| + | | Solubility = Miscible | ||
| + | | SolubleOther = Reacts with [[amine]]s<br>Miscible with [[alcohol]]s<br>Immiscible with hydrocarbons | ||
| + | | Solvent = | ||
| + | | VaporPressure = 0.001 mmHg (20 °C) | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
}} | }} | ||
| Section4 = {{Chembox Thermochemistry | | Section4 = {{Chembox Thermochemistry | ||
| − | | DeltaHf = −814 | + | | DeltaGf = |
| − | | Entropy = 157 | + | | DeltaHc = |
| + | | DeltaHf = −814 kJ·mol<sup>−1</sup> | ||
| + | | Entropy = 157 J·mol<sup>−1</sup>·K<sup>−1</sup> | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
}} | }} | ||
| − | | | + | | Section6 = {{Chembox Hazards |
| − | | ExternalMSDS = [ | + | | AutoignitionPt = Non-flammable |
| − | + | | ExploLimits = | |
| + | | ExternalMSDS = [https://www.fishersci.com/msdsproxy%3FproductName%3DA300700LB%26productDescription%3DSULFURIC%2BAC%2BACS%2B700LB%26catNo%3DA300-700LB%26vendorId%3DVN00033897%26storeId%3D10652 FisherSci] | ||
| FlashPt = Non-flammable | | FlashPt = Non-flammable | ||
| − | | | + | | LD50 = 2.140 mg/kg (rat, oral) |
| − | + | | LC50 = 50 mg/m<sup>3</sup> (guinea pig, 8 hr)<br>510 mg/m<sup>3</sup> (rat, 2 hr)<br>320 mg/m<sup>3</sup> (mouse, 2 hr)<br>18 mg/m<sup>3</sup> (guinea pig) | |
| − | + | | MainHazards = Corrosive | |
| − | + | | NFPA-F = | |
| − | + | | NFPA-H = | |
| − | + | | NFPA-R = | |
| − | + | | NFPA-S = | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | | LC50 = 50 mg/m<sup>3</sup> (guinea pig, 8 hr)<br | + | |
| − | | | + | |
}} | }} | ||
| − | | | + | | Section7 = {{Chembox Related |
| − | | | + | | OtherAnions = |
| − | | | + | | OtherCations = |
| − | | | + | | OtherFunction = |
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Sulfurous acid]]<br>[[Sulfur trioxide]] | ||
}} | }} | ||
}} | }} | ||
| + | '''Sulfuric acid''' (alternative spelling '''sulphuric acid'''), represented by the molecular formula '''H<sub>2</sub>SO<sub>4</sub>''', is one of the most important [[acid]]s in chemistry and the most important chemical to industries in the world. It is the strongest easily available acid, with a pK<sub>a</sub> of -3. | ||
| − | + | ==Properties== | |
| + | ===Chemical properties=== | ||
| + | Sulfuric acid is a diprotic acid, and thus it is able to give away two protons (H<sup>+</sup>). It first dissociates to form [[hydronium]] and hydrogen sulfate/bisulfate ions, with a pK<sub>a</sub> of -3, indicative of a strong acid: | ||
| − | + | : H<sub>2</sub>SO<sub>4</sub> + H<sub>2</sub>O → H<sub>3</sub>O + HSO<sub>4</sub><sup>−</sup> | |
| − | + | The second dissociation forms sulfate and another hydronium ion from a hydrogen sulfate ion. It has a pKa of 1.99, indicative of a mid-strength acid, and occurs like this: | |
| − | + | : HSO<sub>4</sub><sup>−</sup> + H<sub>2</sub>O ⇌ H<sub>3</sub>O<sup>+</sup> + SO<sub>4</sub><sup>2-</sup> | |
| − | + | ||
| − | + | ||
| − | [[ | + | Concentrated sulfuric acid also has a strong oxidizing effect, converting nonmetals such as [[carbon]] and [[sulfur]] to [[carbon dioxide]] and [[sulfur dioxide]], respectively, reducing sulfuric acid into sulfur dioxide and water in the process. |
| − | + | : 2 H<sub>2</sub>SO<sub>4</sub> + C → CO<sub>2</sub> + SO<sub>2</sub> + H<sub>2</sub>O + H<sub>2</sub>SO<sub>4</sub> | |
| − | + | : 2 H<sub>2</sub>SO<sub>4</sub> + S → 2 SO<sub>2</sub> + H<sub>2</sub>O + H<sub>2</sub>SO<sub>4</sub> | |
| − | + | This property is useful for producing large amounts of sulfur dioxide for use as a reducing agent if water is continually removed. Heat accelerates this process. | |
| − | Sulfuric acid | + | Sulfuric acid is sufficiently strong enough to protonate [[nitric acid]], forming the nitronium ion, which can be used in a nitration mixture to make [[alkyl nitrate]]s. |
| − | In | + | In organic chemistry, sulfuric acid is the most practical acid in most cases where a source of H<sub>3</sub>O<sup>+</sup> ions are needed as it introduces the least amount of water. Organic compounds are often easily attacked by the nucleophiles left behind by the dissociation of acids such as HCl which leaves Cl<sup>-</sup> ions behind which can easily attack many organic compounds. However, the [[sulfate]] ions left behind by the dissociation of sulfuric acid are far less reactive than the ions left behind by most acids, it allows to protonate the reaction mixture without causing undesired side reactions in most cases. |
| − | + | When concentrated, it is strongly [[hygroscopy|hygroscopic]] and has strong dehydrating properties. It can break down most organic molecules containing OH<sup>-</sup> groups to use them to form water, leaving only the carbon behind. This property is exploited in the famous [http://youtu.be/w6lfq7BOCik "black snake" demonstration], where sulfuric acid dehydrates [[sucrose]] (table sugar), forming water with the hydrogen and oxygen atoms and leaving amorphous carbon behind. | |
| − | === | + | ===Physical properties=== |
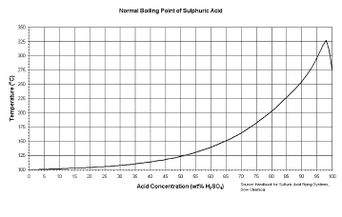
| − | + | [[File:H2so4boil.jpg|thumb|left|342px|Boiling point of H2SO4 VS concentration]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | Sulfuric acid is an oily liquid at room temperature. It is colorless but often has a very light yellow color when slightly contaminated with iron or carbon from organic matter like dust. Even very small amounts of dissolved organic matter can change the color of concentrated sulfuric acid to pale yellow or pink, red, brown, and even black. It is commonly sold diluted at around 35% w/w with water as car battery acid and concentrated between 95% and 98% w/w as drain cleaner. | |
| − | Sulfuric acid | + | Sulfuric acid's boiling point raises with the concentration as described in this figure to the right. An [[azeotrope]] forms at 98% w/w. |
| − | + | At room temperature, sulfuric acid does not fume and has no smell. However, due to its hygroscopicity, closed bottles of conc. sulfuric acid may "smell" harsh, a consequence of inhaling the very dry air from the bottle. Solutions of sulfuric acid may have a weak acidic odor, especially at temperatures higher than room temperature, as a consequence of the solvent vapors carrying tiny amounts of H<sub>2</sub>SO<sub>4</sub> droplets in the air. Hot sulfuric acid is known to fume profusely and smells like a mix of burnt matches and pure pain (this is because of its partial decomposition when hot; the smells correspond to sulfur dioxide and trioxide respectively). | |
| − | + | ==Sources and concentration== | |
| + | ===OTC availability=== | ||
| + | Sulfuric acid is a commonly used chemical for lead-acid batteries and drain cleaning. Battery acid can often be found at an auto store or a department store and is approximately 33-35% sulfuric acid by weight. This is sufficient for most amateur chemists. If more concentrated sulfuric acid is desired, one can look in hardware stores for drain cleaner, which can be over 90% sulfuric acid by weight. For safety purposes, this concentration of sulfuric acid may have a dye in it. Other forms of sulfuric acid may be contaminated with various chemicals and will appear yellow, black, red. | ||
| − | + | For some amateurs, it can be hard to find concentrated sulfuric acid, with acid drain cleaners being banned (as a result of [[wikipedia:Acid_throwing|acid throwing]] or illicit drug manufacture) or very contaminated in some countries. | |
| − | + | As of 2021, concentrated sulfuric acid over 15% is not available in the EU for private individuals, and all conc. sulfuric acid drain cleaners are restricted for professional use only. So far, it's unclear how this affects lead-acid batteries, which require acid in conc. higher than 15%. In certain other countries, 30-36% battery acid is OTC but drain cleaner acid is forbidden; if you happen to live in one of these countries, concentrating sulfuric acid is a must. | |
| − | === | + | ===Concentration=== |
| − | + | The most well-tested method of concentrating sulfuric acid is described in a sub-article: [[Boiling the Bat]]. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | : | + | |
| − | + | ||
| − | + | ||
| − | + | * If you have technical grade sulfuric acid of concentrations from 80% to 94%, it can be converted to the pure compound by Zintl-Karyakin distillation. This process yields sulfuric acid of the highest quality and of concentration above the azeotrope. However, it is demanding in terms of glassware and very risky if performed at home. To perform this distillation, you need [[chromium trioxide]] or a dichromate salt (any will do, ''except ammonium'': [[ammonium dichromate]] will decompose on heating, and you'll have green murky acid contaminated with chromium (III) oxide and chromium sulfate) that will work as an azeotrope breaker. Add the H<sub>2</sub>SO<sub>4</sub>-Cr(VI) mixture to a round-bottom flask, pour the acid in and connect it to an air-cooled condenser. Put thermal insulation ([[asbestos]], rockwool) on the flask and start heating it. Discard the first few grams of the distillate, until its density reaches 1.84; collect every drop after that. This gives pure sulfuric acid with a concentration above 98%. Beware of any spillage of hexavalent chromium, it's a carcinogen! If such a spillage occurs, neutralize it with any reducing solution such as [[sodium thiosulfate]], [[ascorbic acid]] or [[glucose]]. | |
| + | * Simple distillation of conc. drain cleaner sulfuric acid can work on some products, as hot sulfuric acid is oxidizing enough on its own that it will break down many organic contaminants.<ref>https://www.youtube.com/watch?v=4DUGRWjdNLI</ref> Similar to above, discard the first distillate fractions, and only keep the one with a density value of 1.84. This process however, may not work on all drain cleaners, so verify first. | ||
| − | + | It is possible to further concentrate sulfuric acid by adding [[sulfur trioxide]], which reacts with the remaining water to form pure sulfuric acid. Sulfur trioxide can continue to be added to the solution to form [[oleum]], which fumes in air to form sulfuric acid droplets. When an equimolar concentration of sulfuric acid and sulfur trioxide is added, it forms [[pyrosulfuric acid]], which is a solid at room temperature. Sulfur trioxide can easily be obtained through the pyrolysis of certain salts, like anhydrous [[copper(II) sulfate]], [[iron(II) sulfate]], [[sodium pyrosulfate]] or [[potassium persulfate]]. | |
| − | == | + | ==Preparation== |
| + | Sulfuric acid is industrially produced from sulfur, oxygen and water via the conventional contact process (DCDA), lead chamber process<ref>https://www.youtube.com/watch?v=7SDHeTcOXtI</ref> or the wet sulfuric acid process (WSA). The general way these processes work is by burning sulfur to obtain sulfur dioxide, which is oxidized to sulfur trioxide with the help of a catalyst, which in turn is dissolved in concentrated sulfuric acid, to form [[oleum]], which can be further concentrated into and eventually pyrosulfuric acid. The latter two products can be diluted using dil. sulfuric acid into conc. sulfuric acid. Diluted sulfuric acid is preferred instead of pure water, as the dilution is highly exothermic, while the reaction between sulfur trioxide with water is exothermic enough that the resulting sulfuric acid turns into a dense mist. The overall process can be written as: | ||
| − | + | : S + O<sub>2</sub> → SO<sub>2</sub> | |
| − | + | : SO<sub>2</sub> + ½ O<sub>2</sub> → SO<sub>3</sub> | |
| + | : SO<sub>3</sub> + H<sub>2</sub>O → H<sub>2</sub>SO<sub>4</sub> | ||
| + | : SO<sub>3</sub> + H<sub>2</sub>SO<sub>4</sub> → H<sub>2</sub>S<sub>2</sub>O<sub>7</sub> | ||
| + | : H<sub>2</sub>S<sub>2</sub>O<sub>7</sub> + H<sub>2</sub>SO<sub>4</sub> + H<sub>2</sub>O → 3 H<sub>2</sub>SO<sub>4</sub> | ||
| − | + | Each of the three main processes have their own advantages and disadvantages, but in general they work better at large scale, and for the average hobby chemist, while possible to reproduce them at smaller scale, it requires quite a lot of work to make the installation work properly. As such, working with volatile corrosive substances that melt your face off is quite an interesting project, if one were to try. | |
| − | + | There are many other routes to obtain sulfuric acid, most will produce diluted or mildly concentrated solutions, which can be concentrated to obtain more concentrated acid: | |
| − | : | + | *Absorbtion of sulfur dioxide in hydrogen peroxide: hydrogen peroxide will oxidize sulfur dioxide to sulfur trioxide, which reacts immediately with water to form sulfuric acid. Since this reaction is exothermic, an ice bath should be used. If an excess of SO<sub>2</sub> is used, warming the resulting solution to room temperature will cause some of the dissolved gas to boil off as the solution warms.<ref>https://www.youtube.com/watch?v=okvvD3-DF9U</ref> |
| − | + | : H<sub>2</sub>O<sub>2</sub> + SO<sub>2</sub> → H<sub>2</sub>SO<sub>4</sub> | |
| − | + | While very easy to do, this reaction consumes hydrogen peroxide, and since H<sub>2</sub>O<sub>2</sub> is usually available OTC only as solutions from 3% up to 30%, the resulting sulfuric acid will be diluted, requiring further concentration.<ref>https://www.youtube.com/watch?v=mQMj5ier1lY</ref> | |
| − | + | *Oxidation of SO<sub>2</sub> with conc. nitric acid: Similar to the reaction above with H<sub>2</sub>O<sub>2</sub>, conc. nitric acid can be used to oxidize sulfur dioxide directly to sulfuric acid, producing [[nitrogen dioxide]] as side product:<ref>https://www.youtube.com/watch?v=okvvD3-DF9U</ref> | |
| − | : | + | : 2 HNO<sub>3</sub> + SO<sub>2</sub> → H<sub>2</sub>SO<sub>4</sub> + 2 NO<sub>2</sub> |
| − | + | The advantage of this reaction over the one with hydrogen peroxide, is that the nitrogen dioxide can be used to determine when the reaction is complete: when there is not more brown gas being produced, all the nitric acid has been consumed in the reaction. Main disadvantage of this route is that conc. nitric acid is a bit harder to acquire than sulfuric acid, and if one needs conc. sulfuric acid to obtain nitric acid, this route is not suitable. A modification of this reaction can be used, where the resulting nitrogen dioxide gets separated from the reaction, reacted with water to regenerate nitric acid, and then re-added in the reaction flask, to further oxidize the sulfur dioxide. Any nitric oxide produced from the side reaction between sulfur dioxide and nitrogen dioxide, can be reoxidized into nitrogen dioxide by injecting air in the mixture. | |
| − | + | ||
| − | : | + | *Ozone oxidation of sulfur dioxide: Ozone will oxidize sulfur dioxide into sulfur trioxide. This in turn reacts with water to form sulfuric acid. Ozone can be easily made by exposing oxygen to strong UV light, like that one produced by commercial ozone generators or low/high pressure mercury-vapor lamps. If atmospheric air is used, nitrogen dioxide may be produced as side product. This route is attractive since it uses cheap reagents, and while mercury UV lamps are somewhat difficult to properly operate, it's extremely easy to build a contraption where a continuous mixture of sulfur dioxide-oxygen is irradiated by strong UV light in a quartz tube, which produces sulfur trioxide directly. |
| − | + | : 3 O<sub>2</sub> + hv → 2 O<sub>3</sub> | |
| − | : | + | : SO<sub>2</sub> + O<sub>3</sub> → SO<sub>3</sub> + O<sub>2</sub> |
| + | : SO<sub>3</sub> + H<sub>2</sub>O → H<sub>2</sub>SO<sub>4</sub> | ||
| − | + | *Electrolysis of aq. [[copper(II) sulfate]]: In a beaker, a concentrated solution of copper(II) sulfate is added. For cathode, a copper wire is added in the solution, at the bottom, and connected to the negative terminal of a power source, while for anode, a graphite electrode is added in the upper part of the solution, and connected to the positive terminal of the power source. During the process, the copper ions gets deposited on the copper electrode, while oxygen and hydrogen are produced at the carbon electrode. Overall, the reaction is as follows: | |
| − | + | ||
| − | : | + | : CuSO<sub>4</sub> + H<sub>2</sub>O → H<sub>2</sub>SO<sub>4</sub> + Cu + ½ O<sub>2</sub> |
| − | + | The resulting dil. solution of sulfuric acid is purified by filtering it, then concentrated by boiling it. This yields crude conc. H<sub>2</sub>SO<sub>4</sub>, which is distilled off to obtain the pure acid. The process is much easier than other electrochemical routes, as it's clean and relative quickly. Instead of graphite, other electrodes, like lead dioxide, titanium, platinum, or platinum on titanium can also be used.<ref>https://www.youtube.com/watch?v=5dUSF9Gl0xE</ref><ref>https://www.youtube.com/watch?v=ZRYtAquxffE</ref> | |
| − | : | + | *Electrolysis of sulfate salt: This route involves electrolysis of a solution of a soluble sulfate salt, like [[magnesium sulfate]] or even [[ammonium sulfate]], using a diaphragm, which can either be either a classical ion-exchange diaphragm or a flower pot. <ref>https://www.youtube.com/watch?v=6BThiJpbBJQ</ref> The process yields dirty and diluted H<sub>2</sub>SO<sub>4</sub>, which requires purification and concentration.<ref>https://www.youtube.com/watch?v=b2wTha6Z-fA</ref> |
| − | + | *Pyrolysis of pyrosulfates: thermal decomposition of solid pyrosulfates yields sulfate and sulfur trioxide. The resulting sulfur trioxide is absorbed in crushed ice to form sulfuric acid. Further addition of sulfur trioxide yields conc. acid, and if SO<sub>3</sub> keeps getting added, it will convert into oleum, and eventually pyrosulfuric acid. The latter two products can be further diluted to concentrated sulfuric acid, by adding diluted sulfuric acid. For this process, [[sodium pyrosulfate]] is the best material, as it decomposes at a relative low temperature (460 °C) compared to other pyrosulfates, and the compound itself can be made by dehydrating [[sodium bisulfate]], which is readily and cheaply available: | |
| − | + | : 2 NaHSO<sub>4</sub> → Na<sub>2</sub>S<sub>2</sub>O<sub>7</sub> + H<sub>2</sub>O | |
| + | : Na<sub>2</sub>S<sub>2</sub>O<sub>7</sub> → Na<sub>2</sub>SO<sub>4</sub> + SO<sub>3</sub> | ||
| + | : SO<sub>3</sub> + H<sub>2</sub>O → H<sub>2</sub>SO<sub>4</sub> | ||
| − | + | In theory, transition metal sulfates can also be used for this process, but since they decompose at higher temperatures, the resulting sulfur trioxide will partially decompose to sulfur dioxide and oxygen, which may lower the overall yield. | |
| − | : ( | + | *Copper chloride process: in an aqueous solution of [[copper(II) chloride]], sulfur dioxide is bubbled through. This reacts with the CuCl<sub>2</sub> from the aq. solution to form dil. sulfuric acid, HCl and CuCl: |
| − | + | : 2 CuCl<sub>2</sub> + 2 H<sub>2</sub>O + SO<sub>2</sub> → H<sub>2</sub>SO<sub>4</sub> + 2 CuCl + 2 HCl | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | CuCl precipitates out of the solution. By injecting air in the suspension, the CuCl gets reoxidized to CuCl<sub>2</sub>, which can be reused. Sulfur dioxide is reinjected in the solution, which restarts the reaction, then the process gets repeated, until no more SO<sub>2</sub> can absorb in the reaction solution. The yield of this process is not great, unless one uses kg amounts of reagents. Likewise, the oxidation of Cu(I) to Cu(II) using air is very slow, taking many hours, which limits the efficiency of the overall process. | |
| − | + | *Electrobromine process: involves the reaction of elemental sulfur with elemental [[bromine]], using a graphite anode and copper metal cathode. In a beaker, where elemental sulfur is added at the bottom, the two electrodes are introduces, with the graphite electrode resting on the sulfur bed, while the copper anode is only partially submerged in the electrolyte solution. A solution of 5 M [[hydrobromic acid]] is used as electrolyte. When the process is activated, the HBr gets oxidized to bromide ions, which in term convert to elemental bromine, that sink to the bottom, reacting with the sulfur bed to yield disulfur dibromide, which hydrolyzes in water to yield sulfuric acid and HBr, the latter rising back to the anode, where it gets converted back to bromine, and the process repeats. It's important to keep the Cu electrode as high as possible, to prevent the bromide ions from reacting with the elemental bromine, as this yields tribromide ionds, which do not react with the sulfur, and instead just get reduced back into bromide ions, wasting electricity. Eventually, after 1-2 days, the process is almost complete. The solution is filtered off, and the resulting HBr is distilled to be recycled, while the sulfuric acid is concentrated and purified by distillation. The yield of this process is not great, and as it uses bromine, which is highly corrosive and toxic. Likewise, the graphite electrodes get used up very quickly in the reaction. The sulfur bed may break apart during the process, and stirring may be required to break it apart and allow it to settle back. Stop the process and remove the electrodes, before stirring the suspension, and once the sulfur settles back, reintroduce the electrodes, and restart the process. Alternatively, one can a solid piece of sulfur instead of powder, as this shouldn't rise, though this may affect the speed of the reaction, as bulk sulfur reacts slower than powdered sulfur. A porous separating membrane, like a glass fiber cloth may be used to pin the sulfur bed down, while allowing the bromine to diffuse through it to reach the sulfur, though this hasn't been tested so far.<ref>https://www.youtube.com/watch?v=6ms6xbPhdVs</ref> | |
| − | + | ||
| − | + | ||
| − | + | ==Projects== | |
| − | + | * Preparation of metal sulfates | |
| + | * Preparation of nitro compounds through [[nitration]] | ||
| + | * The dehydration of [[sucrose]] to produce elemental [[carbon]] | ||
| + | * [[Esterification]]s that require a dehydrating agent, such as that of [[ethyl acetate]], [[methyl salicylate]], etc. | ||
| + | * Making simple [[rayon]] fibers with [[Schweizer's reagent]] and [[cellulose]] | ||
| + | * Producing other concentrated acids by the reaction of sulfuric acid with an anhydrous salt, such as in the production of fuming [[nitric acid]] and glacial [[acetic acid]] | ||
| − | + | ==Handling== | |
| − | + | ===Safety=== | |
| + | [[File:Corrosive.png|thumb|right|Corrosive]] While low concentration sulfuric acid is relatively safe to work with (under 40% w/w)), concentrated sulfuric acid (over 90% w/w) is extremely corrosive and dangerous. It does not only causes chemical burns, it also causes burns by dehydration of organic materials (like skin), destroying the molecules to form water with the -OH groups in them. Safety measures should be taken and all skin should be covered when working with concentrated sulfuric acid. | ||
| − | + | When heating sulfuric acid, it is important to DO NOT OVERFILL THE FLASK. Concentrated sulfuric acid's volume increases by nearly 16% between 0 and 330°C, an overfilled flask will spill its content. Also, sulfuric acid, even diluted, tends to bump when it boils, accumulating heat to release a violent burst of steam from time to time. The use of boiling chips reduces this phenomenon, but there is no way to stop it completely. It is advised to take measures to prevent spills, an anti-splash adapter with ground glass joint being a very convenient option. | |
| − | + | Hot concentrated sulfuric acid may decompose to form sulfur dioxide and sulfur trioxide, which are toxic and corrosive, respectively. It fumes profusely when hot, the fumes consist of sulfuric acid droplets and a SOx mix. These fumes are very dangerous and a known lung carcinogen. | |
| − | Hot concentrated sulfuric acid | + | |
| − | + | ||
| − | + | ||
| − | + | When carrying glass bottles of sulfuric acid and you worry there's a risk you might break it, a good tip would be to carry it in a (plastic) bucket, partially filled with sand. | |
| − | + | ||
| − | + | ===Storage=== | |
| + | Sulfuric acid should be stored in closed bottles. While glass bottles, being inert, are good for storing concentrated sulfuric acid, concentrated (80-98%) sulfuric acid is often stored in PE (more specifically UDPE or UHDPE) bottles, as PE is not brittle, so in the event you drop the bottle on a hard surface, it will not shatter and splash conc. sulfuric all over the place. Unfortunately, PE bottles are sensitive to light and will degrade over the years if exposed to sunlight, so they must be stored in a dark place away from UV light, like a cupboard. Commercial PE bottles used for conc. sulfuric acids tend to be colored, which helps to limit degradation from strong light and oxygen. However, if you plan to store the acid for more that several years, it's recommended to use glass bottles. | ||
| − | + | Long-term storage of concentrated sulfuric acid may lead to it absorbing water from air and becoming less concentrated. When this happens, the acid needs to be "re-freshened" by distilling unnecessary water off it. If the acid acquired a black or brown color during storage, it needs to be decarbonized: add several drops of concentrated H2O2 to it before distilling off water, the dark color will disappear during heating. | |
| − | + | ||
| − | + | ===Disposal=== | |
| + | Sulfuric acid can be neutralized with any base or carbonate, preferably [[calcium hydroxide]] or carbonate. | ||
| − | + | Concentrated sulfuric acid, like any concentrated acid, should be first strongly dilute it in a large volume of water before neutralizing it with a base. Another method would be to add it in an acid-resistant container with a lid and slowly add solid calcium hydroxide/carbonate or sodium bicarbonate chunks and close the lid to limit splashing. Wait until it stopped fizzing then keep adding until it no longer reacts. Be careful, as the thicker the solution becomes, the stronger the foaming gets. | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | Another method | + | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
==References== | ==References== | ||
| − | + | <references/> | |
| + | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=6911 Sulfuric Acid Production: Revisited] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=2824 H2SO4 by the Lead Chamber Process - success] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=64535 I will now be building and testing my new Batparatus!] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=3722 cleaning sulfuric acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13313 Sulfuric Acid at Home] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=19117 Concentrating dilute sulphuric acid(battery acid) without distillation] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=91332 Sulfuric acid from gypsum using diaphragm cell] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=14857 Sulfuric acid purification] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=14570 sulfuric acid turned black] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=61920 Distilling Sulfuric Acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=65331 Sulfuric acid in NZ] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=14291 Should I get rid of my H2SO4?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13726 sulfuric acid accident] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=62863 Sulfuric acid storage] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13964 HDPE as a storage for Sulfuric Acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13148 Safely Storing H2SO4 (35%)] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=6217 Storage for Sulfuric Acid (H2SO4)] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=25679 Sulfuric Acid and LDPE issue] | ||
| − | + | [[Category:Chemical compounds]] | |
| − | + | [[Category:Inorganic compounds]] | |
| − | + | [[Category:Acids]] | |
| − | + | [[Category:Strong acids]] | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | [[Category: | + | |
| − | [[Category: | + | |
| − | [[Category: | + | |
[[Category:Mineral acids]] | [[Category:Mineral acids]] | ||
| − | [[Category: | + | [[Category:Oxoacids]] |
| + | [[Category:Sulfur oxoacids]] | ||
| + | [[Category:Sulfates]] | ||
[[Category:Oxidizing agents]] | [[Category:Oxidizing agents]] | ||
| − | [[Category: | + | [[Category:Corrosive chemicals]] |
| − | [[Category: | + | [[Category:Materials unstable in basic solution]] |
| − | [[Category: | + | [[Category:Things that can kill you very quickly]] |
| − | [[Category: | + | [[Category:Hygroscopic compounds]] |
| − | [[Category: | + | [[Category:Readily available chemicals]] |
| + | [[Category:Essential reagents]] | ||
| + | [[Category:DEA List II chemicals]] | ||
| + | [[Category:Catalysts]] | ||
| + | [[Category:Liquids]] | ||
Latest revision as of 21:31, 9 September 2023
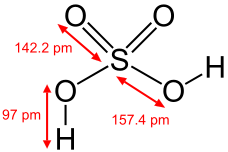 Structure of sulfuric acid
| |
| Names | |
|---|---|
| IUPAC name
Sulfuric acid
| |
| Preferred IUPAC name
Sulfuric acid | |
| Systematic IUPAC name
Sulfuric acid | |
| Other names
Battery acid
Dihydrogen sulfate Oil of vitriol Spirit of vitriol Sulphuric acid | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| H2SO4 | |
| Molar mass | 98.079 g/mol |
| Appearance | Colorless oily liquid |
| Odor | Odorless (air above it may feel dry due to its strong hygroscopicity) |
| Density | 1.84 g/cm3 |
| Melting point | 10 °C (50 °F; 283 K) |
| Boiling point | 337 °C (639 °F; 610 K) (above 300 °C slowly decomposes) |
| Miscible | |
| Solubility | Reacts with amines Miscible with alcohols Immiscible with hydrocarbons |
| Vapor pressure | 0.001 mmHg (20 °C) |
| Acidity (pKa) | −3;1.99 |
| Thermochemistry | |
| Std molar
entropy (S |
157 J·mol−1·K−1 |
| Std enthalpy of
formation (ΔfH |
−814 kJ·mol−1 |
| Hazards | |
| Safety data sheet | FisherSci |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
2.140 mg/kg (rat, oral) |
| LC50 (Median concentration)
|
50 mg/m3 (guinea pig, 8 hr) 510 mg/m3 (rat, 2 hr) 320 mg/m3 (mouse, 2 hr) 18 mg/m3 (guinea pig) |
| Related compounds | |
| Related compounds
|
Sulfurous acid Sulfur trioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfuric acid (alternative spelling sulphuric acid), represented by the molecular formula H2SO4, is one of the most important acids in chemistry and the most important chemical to industries in the world. It is the strongest easily available acid, with a pKa of -3.
Contents
Properties
Chemical properties
Sulfuric acid is a diprotic acid, and thus it is able to give away two protons (H+). It first dissociates to form hydronium and hydrogen sulfate/bisulfate ions, with a pKa of -3, indicative of a strong acid:
- H2SO4 + H2O → H3O + HSO4−
The second dissociation forms sulfate and another hydronium ion from a hydrogen sulfate ion. It has a pKa of 1.99, indicative of a mid-strength acid, and occurs like this:
- HSO4− + H2O ⇌ H3O+ + SO42-
Concentrated sulfuric acid also has a strong oxidizing effect, converting nonmetals such as carbon and sulfur to carbon dioxide and sulfur dioxide, respectively, reducing sulfuric acid into sulfur dioxide and water in the process.
- 2 H2SO4 + C → CO2 + SO2 + H2O + H2SO4
- 2 H2SO4 + S → 2 SO2 + H2O + H2SO4
This property is useful for producing large amounts of sulfur dioxide for use as a reducing agent if water is continually removed. Heat accelerates this process.
Sulfuric acid is sufficiently strong enough to protonate nitric acid, forming the nitronium ion, which can be used in a nitration mixture to make alkyl nitrates.
In organic chemistry, sulfuric acid is the most practical acid in most cases where a source of H3O+ ions are needed as it introduces the least amount of water. Organic compounds are often easily attacked by the nucleophiles left behind by the dissociation of acids such as HCl which leaves Cl- ions behind which can easily attack many organic compounds. However, the sulfate ions left behind by the dissociation of sulfuric acid are far less reactive than the ions left behind by most acids, it allows to protonate the reaction mixture without causing undesired side reactions in most cases.
When concentrated, it is strongly hygroscopic and has strong dehydrating properties. It can break down most organic molecules containing OH- groups to use them to form water, leaving only the carbon behind. This property is exploited in the famous "black snake" demonstration, where sulfuric acid dehydrates sucrose (table sugar), forming water with the hydrogen and oxygen atoms and leaving amorphous carbon behind.
Physical properties
Sulfuric acid is an oily liquid at room temperature. It is colorless but often has a very light yellow color when slightly contaminated with iron or carbon from organic matter like dust. Even very small amounts of dissolved organic matter can change the color of concentrated sulfuric acid to pale yellow or pink, red, brown, and even black. It is commonly sold diluted at around 35% w/w with water as car battery acid and concentrated between 95% and 98% w/w as drain cleaner.
Sulfuric acid's boiling point raises with the concentration as described in this figure to the right. An azeotrope forms at 98% w/w.
At room temperature, sulfuric acid does not fume and has no smell. However, due to its hygroscopicity, closed bottles of conc. sulfuric acid may "smell" harsh, a consequence of inhaling the very dry air from the bottle. Solutions of sulfuric acid may have a weak acidic odor, especially at temperatures higher than room temperature, as a consequence of the solvent vapors carrying tiny amounts of H2SO4 droplets in the air. Hot sulfuric acid is known to fume profusely and smells like a mix of burnt matches and pure pain (this is because of its partial decomposition when hot; the smells correspond to sulfur dioxide and trioxide respectively).
Sources and concentration
OTC availability
Sulfuric acid is a commonly used chemical for lead-acid batteries and drain cleaning. Battery acid can often be found at an auto store or a department store and is approximately 33-35% sulfuric acid by weight. This is sufficient for most amateur chemists. If more concentrated sulfuric acid is desired, one can look in hardware stores for drain cleaner, which can be over 90% sulfuric acid by weight. For safety purposes, this concentration of sulfuric acid may have a dye in it. Other forms of sulfuric acid may be contaminated with various chemicals and will appear yellow, black, red.
For some amateurs, it can be hard to find concentrated sulfuric acid, with acid drain cleaners being banned (as a result of acid throwing or illicit drug manufacture) or very contaminated in some countries.
As of 2021, concentrated sulfuric acid over 15% is not available in the EU for private individuals, and all conc. sulfuric acid drain cleaners are restricted for professional use only. So far, it's unclear how this affects lead-acid batteries, which require acid in conc. higher than 15%. In certain other countries, 30-36% battery acid is OTC but drain cleaner acid is forbidden; if you happen to live in one of these countries, concentrating sulfuric acid is a must.
Concentration
The most well-tested method of concentrating sulfuric acid is described in a sub-article: Boiling the Bat.
- If you have technical grade sulfuric acid of concentrations from 80% to 94%, it can be converted to the pure compound by Zintl-Karyakin distillation. This process yields sulfuric acid of the highest quality and of concentration above the azeotrope. However, it is demanding in terms of glassware and very risky if performed at home. To perform this distillation, you need chromium trioxide or a dichromate salt (any will do, except ammonium: ammonium dichromate will decompose on heating, and you'll have green murky acid contaminated with chromium (III) oxide and chromium sulfate) that will work as an azeotrope breaker. Add the H2SO4-Cr(VI) mixture to a round-bottom flask, pour the acid in and connect it to an air-cooled condenser. Put thermal insulation (asbestos, rockwool) on the flask and start heating it. Discard the first few grams of the distillate, until its density reaches 1.84; collect every drop after that. This gives pure sulfuric acid with a concentration above 98%. Beware of any spillage of hexavalent chromium, it's a carcinogen! If such a spillage occurs, neutralize it with any reducing solution such as sodium thiosulfate, ascorbic acid or glucose.
- Simple distillation of conc. drain cleaner sulfuric acid can work on some products, as hot sulfuric acid is oxidizing enough on its own that it will break down many organic contaminants.[1] Similar to above, discard the first distillate fractions, and only keep the one with a density value of 1.84. This process however, may not work on all drain cleaners, so verify first.
It is possible to further concentrate sulfuric acid by adding sulfur trioxide, which reacts with the remaining water to form pure sulfuric acid. Sulfur trioxide can continue to be added to the solution to form oleum, which fumes in air to form sulfuric acid droplets. When an equimolar concentration of sulfuric acid and sulfur trioxide is added, it forms pyrosulfuric acid, which is a solid at room temperature. Sulfur trioxide can easily be obtained through the pyrolysis of certain salts, like anhydrous copper(II) sulfate, iron(II) sulfate, sodium pyrosulfate or potassium persulfate.
Preparation
Sulfuric acid is industrially produced from sulfur, oxygen and water via the conventional contact process (DCDA), lead chamber process[2] or the wet sulfuric acid process (WSA). The general way these processes work is by burning sulfur to obtain sulfur dioxide, which is oxidized to sulfur trioxide with the help of a catalyst, which in turn is dissolved in concentrated sulfuric acid, to form oleum, which can be further concentrated into and eventually pyrosulfuric acid. The latter two products can be diluted using dil. sulfuric acid into conc. sulfuric acid. Diluted sulfuric acid is preferred instead of pure water, as the dilution is highly exothermic, while the reaction between sulfur trioxide with water is exothermic enough that the resulting sulfuric acid turns into a dense mist. The overall process can be written as:
- S + O2 → SO2
- SO2 + ½ O2 → SO3
- SO3 + H2O → H2SO4
- SO3 + H2SO4 → H2S2O7
- H2S2O7 + H2SO4 + H2O → 3 H2SO4
Each of the three main processes have their own advantages and disadvantages, but in general they work better at large scale, and for the average hobby chemist, while possible to reproduce them at smaller scale, it requires quite a lot of work to make the installation work properly. As such, working with volatile corrosive substances that melt your face off is quite an interesting project, if one were to try.
There are many other routes to obtain sulfuric acid, most will produce diluted or mildly concentrated solutions, which can be concentrated to obtain more concentrated acid:
- Absorbtion of sulfur dioxide in hydrogen peroxide: hydrogen peroxide will oxidize sulfur dioxide to sulfur trioxide, which reacts immediately with water to form sulfuric acid. Since this reaction is exothermic, an ice bath should be used. If an excess of SO2 is used, warming the resulting solution to room temperature will cause some of the dissolved gas to boil off as the solution warms.[3]
- H2O2 + SO2 → H2SO4
While very easy to do, this reaction consumes hydrogen peroxide, and since H2O2 is usually available OTC only as solutions from 3% up to 30%, the resulting sulfuric acid will be diluted, requiring further concentration.[4]
- Oxidation of SO2 with conc. nitric acid: Similar to the reaction above with H2O2, conc. nitric acid can be used to oxidize sulfur dioxide directly to sulfuric acid, producing nitrogen dioxide as side product:[5]
- 2 HNO3 + SO2 → H2SO4 + 2 NO2
The advantage of this reaction over the one with hydrogen peroxide, is that the nitrogen dioxide can be used to determine when the reaction is complete: when there is not more brown gas being produced, all the nitric acid has been consumed in the reaction. Main disadvantage of this route is that conc. nitric acid is a bit harder to acquire than sulfuric acid, and if one needs conc. sulfuric acid to obtain nitric acid, this route is not suitable. A modification of this reaction can be used, where the resulting nitrogen dioxide gets separated from the reaction, reacted with water to regenerate nitric acid, and then re-added in the reaction flask, to further oxidize the sulfur dioxide. Any nitric oxide produced from the side reaction between sulfur dioxide and nitrogen dioxide, can be reoxidized into nitrogen dioxide by injecting air in the mixture.
- Ozone oxidation of sulfur dioxide: Ozone will oxidize sulfur dioxide into sulfur trioxide. This in turn reacts with water to form sulfuric acid. Ozone can be easily made by exposing oxygen to strong UV light, like that one produced by commercial ozone generators or low/high pressure mercury-vapor lamps. If atmospheric air is used, nitrogen dioxide may be produced as side product. This route is attractive since it uses cheap reagents, and while mercury UV lamps are somewhat difficult to properly operate, it's extremely easy to build a contraption where a continuous mixture of sulfur dioxide-oxygen is irradiated by strong UV light in a quartz tube, which produces sulfur trioxide directly.
- 3 O2 + hv → 2 O3
- SO2 + O3 → SO3 + O2
- SO3 + H2O → H2SO4
- Electrolysis of aq. copper(II) sulfate: In a beaker, a concentrated solution of copper(II) sulfate is added. For cathode, a copper wire is added in the solution, at the bottom, and connected to the negative terminal of a power source, while for anode, a graphite electrode is added in the upper part of the solution, and connected to the positive terminal of the power source. During the process, the copper ions gets deposited on the copper electrode, while oxygen and hydrogen are produced at the carbon electrode. Overall, the reaction is as follows:
- CuSO4 + H2O → H2SO4 + Cu + ½ O2
The resulting dil. solution of sulfuric acid is purified by filtering it, then concentrated by boiling it. This yields crude conc. H2SO4, which is distilled off to obtain the pure acid. The process is much easier than other electrochemical routes, as it's clean and relative quickly. Instead of graphite, other electrodes, like lead dioxide, titanium, platinum, or platinum on titanium can also be used.[6][7]
- Electrolysis of sulfate salt: This route involves electrolysis of a solution of a soluble sulfate salt, like magnesium sulfate or even ammonium sulfate, using a diaphragm, which can either be either a classical ion-exchange diaphragm or a flower pot. [8] The process yields dirty and diluted H2SO4, which requires purification and concentration.[9]
- Pyrolysis of pyrosulfates: thermal decomposition of solid pyrosulfates yields sulfate and sulfur trioxide. The resulting sulfur trioxide is absorbed in crushed ice to form sulfuric acid. Further addition of sulfur trioxide yields conc. acid, and if SO3 keeps getting added, it will convert into oleum, and eventually pyrosulfuric acid. The latter two products can be further diluted to concentrated sulfuric acid, by adding diluted sulfuric acid. For this process, sodium pyrosulfate is the best material, as it decomposes at a relative low temperature (460 °C) compared to other pyrosulfates, and the compound itself can be made by dehydrating sodium bisulfate, which is readily and cheaply available:
- 2 NaHSO4 → Na2S2O7 + H2O
- Na2S2O7 → Na2SO4 + SO3
- SO3 + H2O → H2SO4
In theory, transition metal sulfates can also be used for this process, but since they decompose at higher temperatures, the resulting sulfur trioxide will partially decompose to sulfur dioxide and oxygen, which may lower the overall yield.
- Copper chloride process: in an aqueous solution of copper(II) chloride, sulfur dioxide is bubbled through. This reacts with the CuCl2 from the aq. solution to form dil. sulfuric acid, HCl and CuCl:
- 2 CuCl2 + 2 H2O + SO2 → H2SO4 + 2 CuCl + 2 HCl
CuCl precipitates out of the solution. By injecting air in the suspension, the CuCl gets reoxidized to CuCl2, which can be reused. Sulfur dioxide is reinjected in the solution, which restarts the reaction, then the process gets repeated, until no more SO2 can absorb in the reaction solution. The yield of this process is not great, unless one uses kg amounts of reagents. Likewise, the oxidation of Cu(I) to Cu(II) using air is very slow, taking many hours, which limits the efficiency of the overall process.
- Electrobromine process: involves the reaction of elemental sulfur with elemental bromine, using a graphite anode and copper metal cathode. In a beaker, where elemental sulfur is added at the bottom, the two electrodes are introduces, with the graphite electrode resting on the sulfur bed, while the copper anode is only partially submerged in the electrolyte solution. A solution of 5 M hydrobromic acid is used as electrolyte. When the process is activated, the HBr gets oxidized to bromide ions, which in term convert to elemental bromine, that sink to the bottom, reacting with the sulfur bed to yield disulfur dibromide, which hydrolyzes in water to yield sulfuric acid and HBr, the latter rising back to the anode, where it gets converted back to bromine, and the process repeats. It's important to keep the Cu electrode as high as possible, to prevent the bromide ions from reacting with the elemental bromine, as this yields tribromide ionds, which do not react with the sulfur, and instead just get reduced back into bromide ions, wasting electricity. Eventually, after 1-2 days, the process is almost complete. The solution is filtered off, and the resulting HBr is distilled to be recycled, while the sulfuric acid is concentrated and purified by distillation. The yield of this process is not great, and as it uses bromine, which is highly corrosive and toxic. Likewise, the graphite electrodes get used up very quickly in the reaction. The sulfur bed may break apart during the process, and stirring may be required to break it apart and allow it to settle back. Stop the process and remove the electrodes, before stirring the suspension, and once the sulfur settles back, reintroduce the electrodes, and restart the process. Alternatively, one can a solid piece of sulfur instead of powder, as this shouldn't rise, though this may affect the speed of the reaction, as bulk sulfur reacts slower than powdered sulfur. A porous separating membrane, like a glass fiber cloth may be used to pin the sulfur bed down, while allowing the bromine to diffuse through it to reach the sulfur, though this hasn't been tested so far.[10]
Projects
- Preparation of metal sulfates
- Preparation of nitro compounds through nitration
- The dehydration of sucrose to produce elemental carbon
- Esterifications that require a dehydrating agent, such as that of ethyl acetate, methyl salicylate, etc.
- Making simple rayon fibers with Schweizer's reagent and cellulose
- Producing other concentrated acids by the reaction of sulfuric acid with an anhydrous salt, such as in the production of fuming nitric acid and glacial acetic acid
Handling
Safety
While low concentration sulfuric acid is relatively safe to work with (under 40% w/w)), concentrated sulfuric acid (over 90% w/w) is extremely corrosive and dangerous. It does not only causes chemical burns, it also causes burns by dehydration of organic materials (like skin), destroying the molecules to form water with the -OH groups in them. Safety measures should be taken and all skin should be covered when working with concentrated sulfuric acid.When heating sulfuric acid, it is important to DO NOT OVERFILL THE FLASK. Concentrated sulfuric acid's volume increases by nearly 16% between 0 and 330°C, an overfilled flask will spill its content. Also, sulfuric acid, even diluted, tends to bump when it boils, accumulating heat to release a violent burst of steam from time to time. The use of boiling chips reduces this phenomenon, but there is no way to stop it completely. It is advised to take measures to prevent spills, an anti-splash adapter with ground glass joint being a very convenient option.
Hot concentrated sulfuric acid may decompose to form sulfur dioxide and sulfur trioxide, which are toxic and corrosive, respectively. It fumes profusely when hot, the fumes consist of sulfuric acid droplets and a SOx mix. These fumes are very dangerous and a known lung carcinogen.
When carrying glass bottles of sulfuric acid and you worry there's a risk you might break it, a good tip would be to carry it in a (plastic) bucket, partially filled with sand.
Storage
Sulfuric acid should be stored in closed bottles. While glass bottles, being inert, are good for storing concentrated sulfuric acid, concentrated (80-98%) sulfuric acid is often stored in PE (more specifically UDPE or UHDPE) bottles, as PE is not brittle, so in the event you drop the bottle on a hard surface, it will not shatter and splash conc. sulfuric all over the place. Unfortunately, PE bottles are sensitive to light and will degrade over the years if exposed to sunlight, so they must be stored in a dark place away from UV light, like a cupboard. Commercial PE bottles used for conc. sulfuric acids tend to be colored, which helps to limit degradation from strong light and oxygen. However, if you plan to store the acid for more that several years, it's recommended to use glass bottles.
Long-term storage of concentrated sulfuric acid may lead to it absorbing water from air and becoming less concentrated. When this happens, the acid needs to be "re-freshened" by distilling unnecessary water off it. If the acid acquired a black or brown color during storage, it needs to be decarbonized: add several drops of concentrated H2O2 to it before distilling off water, the dark color will disappear during heating.
Disposal
Sulfuric acid can be neutralized with any base or carbonate, preferably calcium hydroxide or carbonate.
Concentrated sulfuric acid, like any concentrated acid, should be first strongly dilute it in a large volume of water before neutralizing it with a base. Another method would be to add it in an acid-resistant container with a lid and slowly add solid calcium hydroxide/carbonate or sodium bicarbonate chunks and close the lid to limit splashing. Wait until it stopped fizzing then keep adding until it no longer reacts. Be careful, as the thicker the solution becomes, the stronger the foaming gets.
References
- ↑ https://www.youtube.com/watch?v=4DUGRWjdNLI
- ↑ https://www.youtube.com/watch?v=7SDHeTcOXtI
- ↑ https://www.youtube.com/watch?v=okvvD3-DF9U
- ↑ https://www.youtube.com/watch?v=mQMj5ier1lY
- ↑ https://www.youtube.com/watch?v=okvvD3-DF9U
- ↑ https://www.youtube.com/watch?v=5dUSF9Gl0xE
- ↑ https://www.youtube.com/watch?v=ZRYtAquxffE
- ↑ https://www.youtube.com/watch?v=6BThiJpbBJQ
- ↑ https://www.youtube.com/watch?v=b2wTha6Z-fA
- ↑ https://www.youtube.com/watch?v=6ms6xbPhdVs
Relevant Sciencemadness threads
- Sulfuric Acid Production: Revisited
- H2SO4 by the Lead Chamber Process - success
- I will now be building and testing my new Batparatus!
- cleaning sulfuric acid
- Sulfuric Acid at Home
- Concentrating dilute sulphuric acid(battery acid) without distillation
- Sulfuric acid from gypsum using diaphragm cell
- Sulfuric acid purification
- sulfuric acid turned black
- Distilling Sulfuric Acid
- Sulfuric acid in NZ
- Should I get rid of my H2SO4?
- sulfuric acid accident
- Sulfuric acid storage
- HDPE as a storage for Sulfuric Acid
- Safely Storing H2SO4 (35%)
- Storage for Sulfuric Acid (H2SO4)
- Sulfuric Acid and LDPE issue
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Inorganic compounds
- Acids
- Strong acids
- Mineral acids
- Oxoacids
- Sulfur oxoacids
- Sulfates
- Oxidizing agents
- Corrosive chemicals
- Materials unstable in basic solution
- Things that can kill you very quickly
- Hygroscopic compounds
- Readily available chemicals
- Essential reagents
- DEA List II chemicals
- Catalysts
- Liquids