Difference between revisions of "Urea"
m (→Reactions) |
Diachrynic (Talk | contribs) (→Preparation) |
||
| (19 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[ | + | {{Chembox |
| − | '''Urea''' or '''carbamide''', is an organic compound commonly used as a fertilizer. It has the chemical formula (NH<sub>2</sub>)<sub>2</sub>CO. Urea was the first organic chemical produced from inorganic chemicals, a breakthrough in chemistry, which proved organic substances can be produced from inorganic substances, disproving the notion of vitalism. | + | | Name = Urea |
| + | | Reference = | ||
| + | | IUPACName = Urea | ||
| + | | PIN = | ||
| + | | SystematicName = Carbonic diamide | ||
| + | | OtherNames = Carbamide<br>Carbonyl diamide<br>Carbonyldiamine<br>Diaminomethanal<br>Diaminomethanone | ||
| + | <!-- Images --> | ||
| + | | ImageFile = Urea.jpg | ||
| + | | ImageSize = 270 | ||
| + | | ImageCaption = Urea crystals obtained from AdBlue | ||
| + | | ImageAlt = | ||
| + | | ImageName = | ||
| + | | ImageFile1 = Urea ball and stick.png | ||
| + | | ImageSize1 = 200 | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageCaption1 = Ball and stick model of urea. | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White solid | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = Decomposes | ||
| + | | Density = 1.32 g/cm<sup>3</sup> | ||
| + | | Formula = CH<sub>4</sub>N<sub>2</sub>O<br>(NH<sub>2</sub>)<sub>2</sub>CO | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 60.06 g/mol | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 133 - 135 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 107.9 g/100 ml (20 °C)<br>167 g/100 ml (40 °C)<br>251 g/100 ml (60 °C)<br>400 g/100 ml (80 °C) | ||
| + | | SolubleOther = Soluble in glacial [[acetic acid]]<br>Insoluble in [[benzene]], [[toluene]], [[xylene]] | ||
| + | | Solubility1 = 0.4 g/100 ml | ||
| + | | Solvent1 = acetonitrile | ||
| + | | Solubility2 = 5 g/100 ml | ||
| + | | Solvent2 = ethanol | ||
| + | | Solubility3 = 50 g/100 ml | ||
| + | | Solvent3 = glycerol | ||
| + | | Solubility4 = 16.6 g/100 ml | ||
| + | | Solvent4 = methanol | ||
| + | | VaporPressure = | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = -47.12 kcal/mol | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = -79.634 kcal/mol | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = [https://www.docdroid.net/X15Opy8/urea-sa.pdf.html Sigma-Aldrich] | ||
| + | | FlashPt = | ||
| + | | LD50 = 8500 mg/kg (oral, rat) | ||
| + | | LC50 = | ||
| + | | MainHazards = Irritant | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[Biuret]] | ||
| + | }} | ||
| + | }} | ||
| + | '''Urea''' or '''carbamide''', is an organic compound commonly used as a fertilizer. It has the chemical formula '''(NH<sub>2</sub>)<sub>2</sub>CO'''. Urea was the first organic chemical produced from inorganic chemicals, a breakthrough in chemistry, which proved organic substances can be produced from inorganic substances, disproving the notion of vitalism. | ||
==Properties== | ==Properties== | ||
| Line 7: | Line 123: | ||
:NH<sub>2</sub>CONH<sub>2</sub> → NH<sub>4</sub>NCO → HNCO + NH<sub>3</sub> | :NH<sub>2</sub>CONH<sub>2</sub> → NH<sub>4</sub>NCO → HNCO + NH<sub>3</sub> | ||
| − | Urea is a weak organic base. It forms salts with strong acids. Despite its two amino groups, it acts as a monoprotic base, only accepting one H+ cation. With diprotic acids such as sulfuric, urea forms two types of salts: monocarbamide dihydrosulfate (carbamonium bisulfate, analogous to inorganic bisulfates) and dicarbamide dihydrosulfate (carbamonium sulfate, analogous to inorganic sulfates). | + | Urea is a weak organic base. It forms salts with strong acids.<ref> |
| + | https://books.google.com/books?id=Y87aAAAAMAAJ&pg=PA12&lpg=PA12&dq=urea+tartrate&source=bl&ots=PYI2nVC-By&sig=7IbwzeNy5bCPdVWiKmzSzQzq7mw&hl=en&sa=X&sqi=2&ved=0ahUKEwimvPTNmKDLAhXCdx4KHaUGDfkQ6AEIMzAE#v=onepage&q=urea%20tartrate&f=false</ref> Despite its two amino groups, it acts as a monoprotic base, only accepting one H+ cation. With diprotic acids such as sulfuric, urea forms two types of salts: monocarbamide dihydrosulfate (carbamonium bisulfate, analogous to inorganic bisulfates) and dicarbamide dihydrosulfate (carbamonium sulfate, analogous to inorganic sulfates). | ||
| − | A frequent contaminant of urea is [[ | + | A frequent contaminant of urea is [[biuret]]. A high concentration of biuret mitigates against using urea as a crop fertilizer, but makes it more valuable as an animal feed additive. |
===Physical=== | ===Physical=== | ||
| − | Urea is a white crystalline substance. Its melting point is between 133-135 °C. Urea is soluble in water (107.9 g/100 ml at 20 °C) and when dissolved, it is neutral on the pH scale. Urea is soluble in [[glycerol]] (500 g/L) and slightly less soluble in [[ethanol]]. | + | Urea is a white crystalline substance. Its melting point is between 133-135 °C. Urea is soluble in water (107.9 g/100 ml at 20 °C) and when dissolved, it is neutral on the pH scale. Urea is soluble in [[glycerol]] (500 g/L) and slightly less soluble in [[ethanol]]. Urea is soluble in [[dimethyl sulfoxide]] (40 g/100 ml at 20-30 °C, 110 g/100 ml at 90-100 °C). <ref>http://www.gaylordchemical.com/uploads/images/pdfs/literature/102B_english.pdf Gaylord Chemical Company</ref> |
==Availability== | ==Availability== | ||
| − | Urea is available as a fertilizer, either pure or mixed with other substances. It can be very cheaply purchased online, often at less than $5/kg. | + | Urea is available as a fertilizer, either pure or mixed with other substances. It can be very cheaply purchased online, often at less than $5/kg. Most of the OTC urea is coated with a special coating which slows its release in the soil, to prevent fertilizer burn. Recrystallization is required to remove this coating. |
[http://www.sciencecompany.com/Urea-500g-P16216.aspx ScienceCompany] sells 500g of lab grade urea at 16.95 $. | [http://www.sciencecompany.com/Urea-500g-P16216.aspx ScienceCompany] sells 500g of lab grade urea at 16.95 $. | ||
Ebay has many listings for urea as a [http://www.ebay.com/sch/Lab-Chemicals-/104233/i.html?_from=R40&_nkw=urea&_sop=15 lab chemical] and as [http://www.ebay.com/sch/Fertilizers-/119683/i.html?_from=R40&_nkw=urea&_sop=15 fertilizer] | Ebay has many listings for urea as a [http://www.ebay.com/sch/Lab-Chemicals-/104233/i.html?_from=R40&_nkw=urea&_sop=15 lab chemical] and as [http://www.ebay.com/sch/Fertilizers-/119683/i.html?_from=R40&_nkw=urea&_sop=15 fertilizer] | ||
| + | |||
| + | AdBlue, used for reduction of nitrous oxide gases from car exhausts, is a quite strong solution of urea in water with little other additives. | ||
==Preparation== | ==Preparation== | ||
Urea was first synthesized by reacting [[silver cyanate]] with [[ammonium chloride]]. | Urea was first synthesized by reacting [[silver cyanate]] with [[ammonium chloride]]. | ||
| − | : | + | :AgOCN + NH<sub>4</sub>Cl → (NH<sub>2</sub>)<sub>2</sub>CO + AgCl |
Industrially it is produced by the thermal decomposition of [[ammonium carbamate]]. | Industrially it is produced by the thermal decomposition of [[ammonium carbamate]]. | ||
| Line 31: | Line 150: | ||
==Reactions== | ==Reactions== | ||
| + | Urea forms many adducts with acids and other inorganic and organic compounds. It also acts as a source of ammonia in certain reactions, such as in the production of [[amide]]s and [[citrazinic acid]]. | ||
{| class="wikitable collapsible collapsed" width="100%" border="0" | {| class="wikitable collapsible collapsed" width="100%" border="0" | ||
!Urea reacts with:|| catalyst|| solvent || product || reaction notes | !Urea reacts with:|| catalyst|| solvent || product || reaction notes | ||
|- style="border=1 cellpadding=2" | |- style="border=1 cellpadding=2" | ||
| − | | [[ | + | | [[Nitric acid]]|| || water || [[urea nitrate]] || exothermic |
| + | |- | ||
| + | | [[Potassium nitrate]] <ref>[http://www.angelfire.com/empire/megapyro6/Pyro/UN.html Making Urea Nitrate without Nitric Acid]</ref> || [[hydrochloric acid]] || water|| [[urea nitrate]] || replacement | ||
| + | |- | ||
| + | | [[Hydrogen peroxide]] || ||water|| [[urea peroxide]] || exothermic | ||
|- | |- | ||
| − | | [[ | + | | [[Citric acid]] <ref>http://link.springer.com/article/10.1007/s11167-005-0579-2#page-1</ref> || || water || [[urea citrate]] || |
|- | |- | ||
| − | | [[ | + | | [[Sodium carbonate]] || || water || [[sodium cyanate]] || produces ammonia gas |
|- | |- | ||
| − | | [[ | + | | [[Malonic acid]] and [[sodium ethoxide]] || ||anhydrous [[ethanol]] || [[barbituric acid]] || condensation |
|} | |} | ||
| Line 48: | Line 172: | ||
*[[Sodium azide]] synthesis | *[[Sodium azide]] synthesis | ||
*[[Hydrazine sulfate]](Hoffman Rearrangement) | *[[Hydrazine sulfate]](Hoffman Rearrangement) | ||
| + | *[[Calcium cyanamide]] synthesis | ||
*Thermal decomposition of molten urea to biuret and ammonia. | *Thermal decomposition of molten urea to biuret and ammonia. | ||
| + | *Make sodium cyanate and [[sodium cyanide]] | ||
==Handling== | ==Handling== | ||
| Line 55: | Line 181: | ||
===Storage=== | ===Storage=== | ||
| − | Urea doesn't require any special storage, and can be safely stored in glass, metal, plastic or ceramic containers. | + | Urea doesn't require any special storage, and can be safely stored in glass, metal, plastic or ceramic containers. Make sure to keep it in a dry place, as urea will absorb water from air and harden itself after a while, if kept in a moist place. |
===Disposal=== | ===Disposal=== | ||
| Line 64: | Line 190: | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
*[http://www.sciencemadness.org/talk/viewthread.php?tid=3386 Urea Synthesis] | *[http://www.sciencemadness.org/talk/viewthread.php?tid=3386 Urea Synthesis] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=63013 Recrystallation of Fertilizer Grade Urea] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
[[Category:Organic compounds]] | [[Category:Organic compounds]] | ||
| + | [[Category:Nitrogen compounds]] | ||
| + | [[Category:Amines]] | ||
[[Category:Bases]] | [[Category:Bases]] | ||
[[Category:Organic bases]] | [[Category:Organic bases]] | ||
| + | [[Category:Ureas]] | ||
[[Category:Readily available chemicals]] | [[Category:Readily available chemicals]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 13:27, 10 July 2022
 Urea crystals obtained from AdBlue
| |
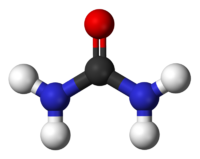 Ball and stick model of urea.
| |
| Names | |
|---|---|
| IUPAC name
Urea
| |
| Systematic IUPAC name
Carbonic diamide | |
| Other names
Carbamide
Carbonyl diamide Carbonyldiamine Diaminomethanal Diaminomethanone | |
| Properties | |
| CH4N2O (NH2)2CO | |
| Molar mass | 60.06 g/mol |
| Appearance | White solid |
| Density | 1.32 g/cm3 |
| Melting point | 133–135 °C (271–275 °F; 406–408 K) |
| Boiling point | Decomposes |
| 107.9 g/100 ml (20 °C) 167 g/100 ml (40 °C) 251 g/100 ml (60 °C) 400 g/100 ml (80 °C) | |
| Solubility | Soluble in glacial acetic acid Insoluble in benzene, toluene, xylene |
| Solubility in acetonitrile | 0.4 g/100 ml |
| Solubility in ethanol | 5 g/100 ml |
| Solubility in glycerol | 50 g/100 ml |
| Solubility in methanol | 16.6 g/100 ml |
| Thermochemistry | |
| Std enthalpy of
formation (ΔfH |
-79.634 kcal/mol |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
8500 mg/kg (oral, rat) |
| Related compounds | |
| Related compounds
|
Biuret |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Urea or carbamide, is an organic compound commonly used as a fertilizer. It has the chemical formula (NH2)2CO. Urea was the first organic chemical produced from inorganic chemicals, a breakthrough in chemistry, which proved organic substances can be produced from inorganic substances, disproving the notion of vitalism.
Contents
Properties
Chemical
Thermal decomposition of urea produces ammonium isocyanate, who in turn yields isocyanuric acid.
- NH2CONH2 → NH4NCO → HNCO + NH3
Urea is a weak organic base. It forms salts with strong acids.[1] Despite its two amino groups, it acts as a monoprotic base, only accepting one H+ cation. With diprotic acids such as sulfuric, urea forms two types of salts: monocarbamide dihydrosulfate (carbamonium bisulfate, analogous to inorganic bisulfates) and dicarbamide dihydrosulfate (carbamonium sulfate, analogous to inorganic sulfates).
A frequent contaminant of urea is biuret. A high concentration of biuret mitigates against using urea as a crop fertilizer, but makes it more valuable as an animal feed additive.
Physical
Urea is a white crystalline substance. Its melting point is between 133-135 °C. Urea is soluble in water (107.9 g/100 ml at 20 °C) and when dissolved, it is neutral on the pH scale. Urea is soluble in glycerol (500 g/L) and slightly less soluble in ethanol. Urea is soluble in dimethyl sulfoxide (40 g/100 ml at 20-30 °C, 110 g/100 ml at 90-100 °C). [2]
Availability
Urea is available as a fertilizer, either pure or mixed with other substances. It can be very cheaply purchased online, often at less than $5/kg. Most of the OTC urea is coated with a special coating which slows its release in the soil, to prevent fertilizer burn. Recrystallization is required to remove this coating.
ScienceCompany sells 500g of lab grade urea at 16.95 $.
Ebay has many listings for urea as a lab chemical and as fertilizer
AdBlue, used for reduction of nitrous oxide gases from car exhausts, is a quite strong solution of urea in water with little other additives.
Preparation
Urea was first synthesized by reacting silver cyanate with ammonium chloride.
- AgOCN + NH4Cl → (NH2)2CO + AgCl
Industrially it is produced by the thermal decomposition of ammonium carbamate.
It can also be produced from the reaction of phosgene and ammonia
- COCl2 + 4 NH3 → (NH2)2CO + 2 NH4Cl
Reactions
Urea forms many adducts with acids and other inorganic and organic compounds. It also acts as a source of ammonia in certain reactions, such as in the production of amides and citrazinic acid.
| Urea reacts with: | catalyst | solvent | product | reaction notes |
|---|---|---|---|---|
| Nitric acid | water | urea nitrate | exothermic | |
| Potassium nitrate [3] | hydrochloric acid | water | urea nitrate | replacement |
| Hydrogen peroxide | water | urea peroxide | exothermic | |
| Citric acid [4] | water | urea citrate | ||
| Sodium carbonate | water | sodium cyanate | produces ammonia gas | |
| Malonic acid and sodium ethoxide | anhydrous ethanol | barbituric acid | condensation |
Projects
- Urea nitrate synthesis
- Sodium azide synthesis
- Hydrazine sulfate(Hoffman Rearrangement)
- Calcium cyanamide synthesis
- Thermal decomposition of molten urea to biuret and ammonia.
- Make sodium cyanate and sodium cyanide
Handling
Safety
Urea is non-toxic, though is may cause irritations to sensitive tissues. It may corrode metallic surfaces, even the more resistant forms of stainless steel.
Storage
Urea doesn't require any special storage, and can be safely stored in glass, metal, plastic or ceramic containers. Make sure to keep it in a dry place, as urea will absorb water from air and harden itself after a while, if kept in a moist place.
Disposal
As urea is a good fertilizer, it can be safely dumped in the soil, although it's recommended to avoid dumping it in water reservoirs to prevent the growth on unwanted algae. It can also be used to as a nitrogen source for aerobic bacteria in the conversion of sawdust to compost.
References
- ↑ https://books.google.com/books?id=Y87aAAAAMAAJ&pg=PA12&lpg=PA12&dq=urea+tartrate&source=bl&ots=PYI2nVC-By&sig=7IbwzeNy5bCPdVWiKmzSzQzq7mw&hl=en&sa=X&sqi=2&ved=0ahUKEwimvPTNmKDLAhXCdx4KHaUGDfkQ6AEIMzAE#v=onepage&q=urea%20tartrate&f=false
- ↑ http://www.gaylordchemical.com/uploads/images/pdfs/literature/102B_english.pdf Gaylord Chemical Company
- ↑ Making Urea Nitrate without Nitric Acid
- ↑ http://link.springer.com/article/10.1007/s11167-005-0579-2#page-1