Nicotine
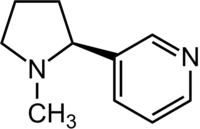
| |
| Names | |
|---|---|
| IUPAC name
(S)-3-[1-Methylpyrrolidin-2-yl]pyridine
| |
| Other names
Nicorette
Nicotrol | |
| Identifiers | |
| Jmol-3D images | Image |
| |
| Properties | |
| C10H14N2 | |
| Molar mass | 162.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Weak amine-like, stronger at high temperatures |
| Density | 1.01 g/cm3 |
| Melting point | −79 °C (−110 °F; 194 K) |
| Boiling point | 247 °C (477 °F; 520 K) |
| Miscible | |
| Solubility | Very soluble in alcohol, chloroform, diethyl ether, kerosene, oils, petroleum ether |
| Vapor pressure | 0.038 mmHg at 25 °C |
| Hazards | |
| Safety data sheet | Sigma-Aldrich (±) |
| Flash point | 95 °C (203 °F; 368 K) |
| Related compounds | |
| Related compounds
|
Niacin |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nicotine is an alkaloid found in the nightshade family of plants (Solanaceae), such as tobacco. It is largely used as an insecticide and can be found in many common products, such as cigarettes. It has the formula C10H14N2.
Contents
Properties
Chemical
On exposure to UV light or various oxidizing agents, nicotine is converted to nicotinic acid (vitamin B3), nicotine oxide, and methylamine. As the active stimulant in tobacco products, it is a highly addictive drug when absorbed and exhibits neurotoxic properties in the body.
As a nitrogenous base, nicotine forms salts with acids, that are usually solid and water-soluble.
Physical
Nicotine is a hygroscopic, colorless oily liquid, miscible in alcohol, ether or light petroleum. It is also soluble in water in its base form. Impure nicotine has a brownish color, due to tar and other organic substances dissolved. Nicotine's flash point is 95 °C and the auto-ignition temperature is 244 °C. Nicotine melts at -79 °C and boils at 247 °C. It has a density of 1.01 g/cm3.[1]
Availability
Nicotine is available in the plants of the nightshade family, in various concentrations, mostly in leaves. In case of tobacco, it constitutes approximately 0.6–3.5% of the dry weight and is present in the range of 2–7 µg/kg of various edible plants.
Several insecticides contain nicotine salts, such as nicotine chloride or sulfate.
Nicotine patches contain a small amount of nicotine.
Electronic cigarette stores will sell diluted glycerol solutions of nicotine. However, due to the high boiling point of both solvents and their tendency to decompose before their boiling point, extracting the nicotine from the solution is impractical.
In most localities, it is illegal to buy tobacco as a minor or buy tobacco for a minor.
Preparation and isolation
Nicotine can be extracted from tobacco leaves or any other plant from its family. As it is water-soluble, nicotine is easy to extract, simply boiling tobacco leaves in water will cause the nicotine from the plant to dissolve in the boiling water. However, it's difficult to purify it, as nicotine is also a solvent and may dissolve many other alkaloids from tobacco that are not water-soluble.
One method of extracting relative pure nicotine involves the steam distillation of tobacco leaves from a sodium hydroxide solution, as seen in this video.
Projects
- Nicotine-based insecticides
- Niacin (nicotinic acid aka Vitamin B3) synthesis
Handling
Safety
Concentrated nicotine is highly toxic and quantities as small as 60-100 mg can cause death for an average non-smoker. Smokers can tolerate a slightly higher dose. Nicotine can easily be absorbed through the skin, making it a contact poison, and should be treated as such. Proper protection, such as gloves, water-proof apron, and goggles should be worn when handling the compound, and a gas mask may be necessary if it is fine enough to become airborne.
Storage
Nicotine should be stored in small quantities in closed containers, in dark well ventilated areas.
Disposal
Nicotine is biodegradable. Its decomposition is accelerated with strong UV light.
Burning it may not be an option, as it has a high flash point (95 °C) and the resulting smoke contains plenty of unburnt nicotine.
Heating nicotine with elemental selenium will destroy the compound.
Diluted nicotine can be used as an insecticide, as long as it's not contaminated with harmful non-tobacco extracts. Plant-derivative nicotine is safe to use.
References
Sciencemadness library
Relevant Sciencemadness threads
- Chemical pages without CAS Registry Number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemical compounds
- Organic compounds
- Biologically-derived compounds
- Things that can kill you very quickly
- Aromatic compounds
- Neurotoxins
- Contact poisons
- Psychoactive substances
- Liquids