Difference between revisions of "Acetic acid"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
| OtherNames = Essence of vinegar (concentrated)<br>Hydrogen acetate<br>Methanecarboxylic acid<br>Vinegar (dilute) | | OtherNames = Essence of vinegar (concentrated)<br>Hydrogen acetate<br>Methanecarboxylic acid<br>Vinegar (dilute) | ||
<!-- Images --> | <!-- Images --> | ||
| − | | ImageFile = | + | | ImageFile = Acetic acid glacial bottle and sample.jpg |
| − | | ImageSize = | + | | ImageSize = 300 |
| ImageAlt = | | ImageAlt = | ||
| ImageName = | | ImageName = | ||
| + | | ImageCaption = Glacial acetic acid | ||
| ImageFile1 = | | ImageFile1 = | ||
| ImageSize1 = | | ImageSize1 = | ||
| Line 67: | Line 68: | ||
| pKb = 9.24 (acetate ion) | | pKb = 9.24 (acetate ion) | ||
| Solubility = Miscible | | Solubility = Miscible | ||
| − | | SolubleOther = Reacts with amines and bases<br>Miscible with [[acetone]], [[benzene]], [[diethyl ether]], [[ethanol]], [[glycerol]], [[isopropanol]], [[methanol]]<br>Immiscible with [[carbon disulfide]], [[carbon tetrachloride | + | | SolubleOther = Reacts with amines and bases<br>Miscible with [[acetone]], [[benzene]], [[diethyl ether]], [[ethanol]], [[glycerol]], [[hexane]], [[isopropanol]], [[methanol]]<br>Immiscible with [[carbon disulfide]], [[carbon tetrachloride]], [[pentane]] |
| Solvent = | | Solvent = | ||
| VaporPressure = 11.4 mmHg at 20 °C | | VaporPressure = 11.4 mmHg at 20 °C | ||
| Line 113: | Line 114: | ||
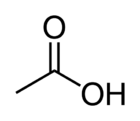
'''Acetic acid''' (or '''ethanoic acid''') is an organic compound with the chemical formula '''CH<sub>3</sub>COOH'''. It is a colorless liquid that when undiluted is called ''glacial acetic acid''. | '''Acetic acid''' (or '''ethanoic acid''') is an organic compound with the chemical formula '''CH<sub>3</sub>COOH'''. It is a colorless liquid that when undiluted is called ''glacial acetic acid''. | ||
| − | Vinegar is roughly 4%-8% acetic acid by volume, and its characteristic smell and taste is due to acetic acid. Although it is classified as a weak acid, concentrated acetic acid is corrosive and can attack the skin. | + | ''Vinegar'' is roughly 4%-8% acetic acid by volume, and its characteristic smell and taste is due to acetic acid. Although it is classified as a weak acid, concentrated acetic acid is corrosive and can attack the skin. |
==Properties== | ==Properties== | ||
===Physical=== | ===Physical=== | ||
| − | Acetic acid is a transparent, colorless liquid at room temperature with a slightly higher viscosity than water solidifying at about 10 °C and boiling at 117 to 118° C. It has a pungent odor and sour, acidic taste. Acetic acid is a | + | Acetic acid is a transparent, colorless liquid at room temperature with a slightly higher viscosity than water solidifying at about 10 °C and boiling at 117 to 118° C. It has a pungent odor and sour, acidic taste. Acetic acid is a polar protic solvent, similar to ethanol and water, but unlike water, acetic acid can dissolve not only polar compounds such as inorganic salts and sugars, but also non-polar compounds such as oils and elements such as sulfur and iodine. It readily mixes with other polar and non-polar solvents such as water, [[chloroform]], and [[hexane]]. With higher [[alkane]]s (starting with [[octane]]), acetic acid is not completely miscible anymore, and its miscibility continues to decline with longer n-alkanes. |
This dissolving property and miscibility of acetic acid makes it a widely used industrial chemical, for example, as a solvent in the production of dimethyl terephthalate. | This dissolving property and miscibility of acetic acid makes it a widely used industrial chemical, for example, as a solvent in the production of dimethyl terephthalate. | ||
| Line 128: | Line 129: | ||
With superbases (e.g., organolithium reagents), it can be doubly deprotonated to give LiCH<sub>2</sub>CO<sub>2</sub>Li. Reduction of acetic acid gives [[ethanol]]. | With superbases (e.g., organolithium reagents), it can be doubly deprotonated to give LiCH<sub>2</sub>CO<sub>2</sub>Li. Reduction of acetic acid gives [[ethanol]]. | ||
| − | When heated above 440 °C (824 °F), acetic acid decomposes to produce [[methane]] and [[carbon dioxide]], or [[ketene]] and water: | + | When heated above 440 °C (824 °F), acetic acid decomposes to produce [[methane]] and [[carbon dioxide]], or [[ethenone|ketene]] and water: |
:CH<sub>3</sub>COOH → CH<sub>4</sub> + CO<sub>2</sub> | :CH<sub>3</sub>COOH → CH<sub>4</sub> + CO<sub>2</sub> | ||
| Line 136: | Line 137: | ||
:Mg + 2 CH<sub>3</sub>COOH → Mg(CH<sub>3</sub>COO)<sub>2</sub> + H<sub>2</sub> | :Mg + 2 CH<sub>3</sub>COOH → Mg(CH<sub>3</sub>COO)<sub>2</sub> + H<sub>2</sub> | ||
| − | :Fe + 2 CH<sub>3</sub>COOH → | + | :Fe + 2 CH<sub>3</sub>COOH → Fe(CH<sub>3</sub>COO)<sub>2</sub> + H<sub>2</sub> |
| − | :Zn + 2 CH<sub>3</sub>COOH → | + | :Zn + 2 CH<sub>3</sub>COOH → Zn(CH<sub>3</sub>COO)<sub>2</sub> + H<sub>2</sub> |
When mixed with a sufficient oxidizing agent, usually hydrogen peroxide, acetic acid can be reacted directly with less reactive metals (such as [[copper]]) to yield an acetate, though more hydrogen peroxide will need to be added to continue the process. Copper acetate can be made this way. Acetates can also be prepared from acetic acid and an appropriate carbonate or hydoxide as in the popular reaction with [[Sodium bicarbonate|baking soda]]: | When mixed with a sufficient oxidizing agent, usually hydrogen peroxide, acetic acid can be reacted directly with less reactive metals (such as [[copper]]) to yield an acetate, though more hydrogen peroxide will need to be added to continue the process. Copper acetate can be made this way. Acetates can also be prepared from acetic acid and an appropriate carbonate or hydoxide as in the popular reaction with [[Sodium bicarbonate|baking soda]]: | ||
| Line 144: | Line 145: | ||
Because [[aluminium]] forms a [[Passivation|passivating]] acid-resistant film of [[aluminium oxide]], aluminium tanks are used to transport acetic acid. | Because [[aluminium]] forms a [[Passivation|passivating]] acid-resistant film of [[aluminium oxide]], aluminium tanks are used to transport acetic acid. | ||
| + | |||
| + | Acetic acid will react with alcohols in the presence of a catalyst, such as [[sulfuric acid]] to form [[ester]]s: | ||
| + | |||
| + | :CH<sub>3</sub>COOH + R-OH → CH<sub>3</sub>C(=O)O-R + H<sub>2</sub>O | ||
| + | |||
| + | Acetic acid reacts with chlorodehydrating agents, like [[phosphorus trichloride]]/[[phosphorus pentachloride|pentachloride]], [[thionyl chloride]], [[sulfuryl chloride]] or [[phosgene]] to give [[acetyl chloride]]: | ||
| + | |||
| + | :3 CH<sub>3</sub>COOH + PCl<sub>3</sub> → CH<sub>3</sub>COCl + 3 HCl + H<sub>3</sub>PO<sub>4</sub> | ||
| + | :5 CH<sub>3</sub>COOH + PCl<sub>5</sub> → CH<sub>3</sub>COCl + 5 HCl + H<sub>3</sub>PO<sub>4</sub> | ||
| + | :2 CH<sub>3</sub>COOH + SOCl<sub>2</sub> → CH<sub>3</sub>COCl + HCl + SO<sub>2</sub> | ||
| + | :2 CH<sub>3</sub>COOH + SO<sub>2</sub>Cl<sub>2</sub> → CH<sub>3</sub>COCl + 2 HCl + SO<sub>2</sub> | ||
| + | :2 CH<sub>3</sub>COOH + COCl<sub>2</sub> → CH<sub>3</sub>COCl + 2 HCl + CO<sub>2</sub> | ||
==Availability== | ==Availability== | ||
| − | [[File:Stop bath.jpg|thumb| | + | [[File:Stop bath.jpg|thumb|240px]] |
While distilled vinegar is the most readily available form of acetic acid, due to its low concentration and the large amount of space it requires, other sources are needed for efficient use as a reagent. A good source of food grade acetic acid is vinegar essence which has a concentration between 20-80% acetic acid, depending on the brand and can be found in many stores, although in some countries it has become hard to find in recent years. | While distilled vinegar is the most readily available form of acetic acid, due to its low concentration and the large amount of space it requires, other sources are needed for efficient use as a reagent. A good source of food grade acetic acid is vinegar essence which has a concentration between 20-80% acetic acid, depending on the brand and can be found in many stores, although in some countries it has become hard to find in recent years. | ||
| − | Acetic acid is also used in photographic stop baths, which consists of high concentrations of buffered acetic acid, often with a pH indicator included. These can be removed through distillation. Glacial acetic acid can be purchased from online [[Lab suppliers|suppliers]] for modest prices as well. | + | Acetic acid is also used in photographic stop baths, which consists of high concentrations of buffered acetic acid, often with a pH indicator included. These can be removed through distillation. Glacial acetic acid can be purchased from online [[Lab suppliers|suppliers]] for modest prices as well. |
| + | |||
| + | Acetic acid is also sold by some beekeeper stores, in various concentrations, such as 60%, 80% and 100%. | ||
Distilled vinegar is not distilled acetic acid. In the production of distilled vinegar, the starting ingredients are distilled, NOT the final product. There are significant amounts of organic contaminants in the product, which can be observed to decompose on heating. [[Sodium acetate]] made with baking soda and distilled vinegar and boiled to dryness tends to be brown, not clear white. | Distilled vinegar is not distilled acetic acid. In the production of distilled vinegar, the starting ingredients are distilled, NOT the final product. There are significant amounts of organic contaminants in the product, which can be observed to decompose on heating. [[Sodium acetate]] made with baking soda and distilled vinegar and boiled to dryness tends to be brown, not clear white. | ||
| Line 159: | Line 174: | ||
*Make acetate esters such as [[methyl acetate]] and [[ethyl acetate]] | *Make acetate esters such as [[methyl acetate]] and [[ethyl acetate]] | ||
*Make sodium or calcium acetate | *Make sodium or calcium acetate | ||
| + | *Remove rust | ||
| + | *Sterilize beehives | ||
| + | *Cooking (vinegar, food-grade) | ||
==Handling== | ==Handling== | ||
| Line 168: | Line 186: | ||
===Disposal=== | ===Disposal=== | ||
| − | Acetic acid can be neutralized with a base or a carbonate, such as calcium carbonate. | + | Acetic acid can be neutralized with a base or a carbonate, such as [[calcium carbonate]]. Concentrated acid should be diluted first, before neutralization to prevent splashing. |
==References== | ==References== | ||
| Line 175: | Line 193: | ||
*Chemical Reagents Their Purity and Tests By E. Merck | *Chemical Reagents Their Purity and Tests By E. Merck | ||
===Relevant Sciencemadness threads=== | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=12898 Making glacial acetic acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=4122 Glacial acetic acid...] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=75811 OTC glacial acetic acid need help] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=29096 Preparation of acetic acid?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=29728 Acetic Acid Synth. Easy Home Version.] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=7775 purifying acetic acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13818 White vinegar to acetic acid] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=23404 Purification Of Acetic Acid From Vinegar] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=75225 Glacial acetic acid from acetic anhydride?] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=13135 Glacial Acetic Acid synthesis wo sulfuric] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=69109 Acetic acid from acetone] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=78725 Glacial acetic acid dillution to 80%,from 99.9%] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=32307 Acetic Acid Distillation with an Entrainer] | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=8794 purify acetic acid] | ||
[[Category:Chemical compounds]] | [[Category:Chemical compounds]] | ||
| Line 184: | Line 216: | ||
[[Category:Polar solvents]] | [[Category:Polar solvents]] | ||
[[Category:Protic solvents]] | [[Category:Protic solvents]] | ||
| − | |||
| − | |||
[[Category:Volatile chemicals]] | [[Category:Volatile chemicals]] | ||
[[Category:Liquids]] | [[Category:Liquids]] | ||
[[Category:Irritants]] | [[Category:Irritants]] | ||
| + | [[Category:Readily available chemicals]] | ||
| + | [[Category:Materials available as food grade]] | ||
| + | [[Category:Essential reagents]] | ||
Latest revision as of 14:43, 18 November 2023
 Glacial acetic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Acetic acid
| |||
| Systematic IUPAC name
Ethanoic acid | |||
| Other names
Essence of vinegar (concentrated)
Hydrogen acetate Methanecarboxylic acid Vinegar (dilute) | |||
| Properties | |||
| C2H4O2 CH3COOH | |||
| Molar mass | 60.053 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent, vinegar-like | ||
| Density | 1.049 g/cm3 | ||
| Melting point | 16.6 °C (61.9 °F; 289.8 K) | ||
| Boiling point | 117.9 °C (244.2 °F; 391.0 K) | ||
| Miscible | |||
| Solubility | Reacts with amines and bases Miscible with acetone, benzene, diethyl ether, ethanol, glycerol, hexane, isopropanol, methanol Immiscible with carbon disulfide, carbon tetrachloride, pentane | ||
| Vapor pressure | 11.4 mmHg at 20 °C | ||
| Acidity (pKa) | 4.76 | ||
| Viscosity | 1.22 | ||
| Thermochemistry | |||
| Std molar
entropy (S |
158.0 J·K−1·mol−1 | ||
| Std enthalpy of
formation (ΔfH |
-483.88—483.16 kJ/mol | ||
| Hazards | |||
| Safety data sheet | Sigma-Aldrich | ||
| Flash point | 40 °C (104 °F; 313 K) | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (Median dose)
|
3,310 mg/kg (rat, oral) | ||
| LC50 (Median concentration)
|
5,620 ppm (mouse, 1 hr) 16,000 ppm (rat, 4 hr) | ||
| Related compounds | |||
| Related compounds
|
Formic acid Propionic acid Butyric acid | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Acetic acid (or ethanoic acid) is an organic compound with the chemical formula CH3COOH. It is a colorless liquid that when undiluted is called glacial acetic acid.
Vinegar is roughly 4%-8% acetic acid by volume, and its characteristic smell and taste is due to acetic acid. Although it is classified as a weak acid, concentrated acetic acid is corrosive and can attack the skin.
Contents
Properties
Physical
Acetic acid is a transparent, colorless liquid at room temperature with a slightly higher viscosity than water solidifying at about 10 °C and boiling at 117 to 118° C. It has a pungent odor and sour, acidic taste. Acetic acid is a polar protic solvent, similar to ethanol and water, but unlike water, acetic acid can dissolve not only polar compounds such as inorganic salts and sugars, but also non-polar compounds such as oils and elements such as sulfur and iodine. It readily mixes with other polar and non-polar solvents such as water, chloroform, and hexane. With higher alkanes (starting with octane), acetic acid is not completely miscible anymore, and its miscibility continues to decline with longer n-alkanes.
This dissolving property and miscibility of acetic acid makes it a widely used industrial chemical, for example, as a solvent in the production of dimethyl terephthalate.
Chemical
Acetic acid undergoes the typical chemical reactions of a carboxylic acid. Upon treatment with a standard base, it converts to metal acetate and water:
- CH3COOH + MOH → CH3COOM + H2
With superbases (e.g., organolithium reagents), it can be doubly deprotonated to give LiCH2CO2Li. Reduction of acetic acid gives ethanol.
When heated above 440 °C (824 °F), acetic acid decomposes to produce methane and carbon dioxide, or ketene and water:
- CH3COOH → CH4 + CO2
- CH3COOH → CH2CO + H2O
Acetic acid is mildly corrosive to metals including magnesium, iron and zinc, forming hydrogen gas and metal acetates.
- Mg + 2 CH3COOH → Mg(CH3COO)2 + H2
- Fe + 2 CH3COOH → Fe(CH3COO)2 + H2
- Zn + 2 CH3COOH → Zn(CH3COO)2 + H2
When mixed with a sufficient oxidizing agent, usually hydrogen peroxide, acetic acid can be reacted directly with less reactive metals (such as copper) to yield an acetate, though more hydrogen peroxide will need to be added to continue the process. Copper acetate can be made this way. Acetates can also be prepared from acetic acid and an appropriate carbonate or hydoxide as in the popular reaction with baking soda:
- NaHCO3 + CH3COOH → CH3COONa + CO2 + H2O
Because aluminium forms a passivating acid-resistant film of aluminium oxide, aluminium tanks are used to transport acetic acid.
Acetic acid will react with alcohols in the presence of a catalyst, such as sulfuric acid to form esters:
- CH3COOH + R-OH → CH3C(=O)O-R + H2O
Acetic acid reacts with chlorodehydrating agents, like phosphorus trichloride/pentachloride, thionyl chloride, sulfuryl chloride or phosgene to give acetyl chloride:
- 3 CH3COOH + PCl3 → CH3COCl + 3 HCl + H3PO4
- 5 CH3COOH + PCl5 → CH3COCl + 5 HCl + H3PO4
- 2 CH3COOH + SOCl2 → CH3COCl + HCl + SO2
- 2 CH3COOH + SO2Cl2 → CH3COCl + 2 HCl + SO2
- 2 CH3COOH + COCl2 → CH3COCl + 2 HCl + CO2
Availability
While distilled vinegar is the most readily available form of acetic acid, due to its low concentration and the large amount of space it requires, other sources are needed for efficient use as a reagent. A good source of food grade acetic acid is vinegar essence which has a concentration between 20-80% acetic acid, depending on the brand and can be found in many stores, although in some countries it has become hard to find in recent years.
Acetic acid is also used in photographic stop baths, which consists of high concentrations of buffered acetic acid, often with a pH indicator included. These can be removed through distillation. Glacial acetic acid can be purchased from online suppliers for modest prices as well.
Acetic acid is also sold by some beekeeper stores, in various concentrations, such as 60%, 80% and 100%.
Distilled vinegar is not distilled acetic acid. In the production of distilled vinegar, the starting ingredients are distilled, NOT the final product. There are significant amounts of organic contaminants in the product, which can be observed to decompose on heating. Sodium acetate made with baking soda and distilled vinegar and boiled to dryness tends to be brown, not clear white.
Preparation
One of the most common syntheses for acetic acid discussed involves the acidification of an excess of an acetate salt to yield acetic acid. This somewhat impractical method requires the addition of concentrated sulfuric acid to anhydrous calcium acetate or sodium acetate followed by a second distillation over an anhydrous hygroscopic salt to remove water. The acetate salt in this method is easily obtained by the combination of household vinegar with a base and subsequent crystallization of the reacted solution, but this is a very tedious process for producing larger amounts of the acid.
Projects
- Make acetate esters such as methyl acetate and ethyl acetate
- Make sodium or calcium acetate
- Remove rust
- Sterilize beehives
- Cooking (vinegar, food-grade)
Handling
Safety
Diluted acetic acid, aka vinegar, is irritant to nose and mouth, while glacial acetic acid is corrosive. Otherwise it is not particularly toxic.
Storage
Storage for glacial or concentrated acetic acid should be done in closed bottles, away from any rust susceptible metals, best in an acid cabinet.
Disposal
Acetic acid can be neutralized with a base or a carbonate, such as calcium carbonate. Concentrated acid should be diluted first, before neutralization to prevent splashing.
References
- http://en.wikipedia.org/wiki/Acetic_acid
- Chemical Reagents Their Purity and Tests By E. Merck
Relevant Sciencemadness threads
- Making glacial acetic acid
- Glacial acetic acid...
- OTC glacial acetic acid need help
- Preparation of acetic acid?
- Acetic Acid Synth. Easy Home Version.
- purifying acetic acid
- White vinegar to acetic acid
- Purification Of Acetic Acid From Vinegar
- Glacial acetic acid from acetic anhydride?
- Glacial Acetic Acid synthesis wo sulfuric
- Acetic acid from acetone
- Glacial acetic acid dillution to 80%,from 99.9%
- Acetic Acid Distillation with an Entrainer
- purify acetic acid